Neonates and infants aged <8 months whose mothers were not vaccinated at least 2 weeks before giving birth, or who are at increased risk of severe disease, are recommended to receive passive immunisation with a long-acting RSV-specific monoclonal antibody
A single dose of the RSV vaccine Abrysvo is recommended for all pregnant women from 28 weeks gestation, as the first opportunity to protect their infant. See Pregnant women are recommended to receive an RSV vaccine during pregnancy to protect the infant. Do not give RSV vaccine Abrysvo to infants and young children.
Nirsevimab is a long-acting RSV-specific monoclonal antibody that is recommended for infants who were born:
- to women who did not receive RSV vaccine during pregnancy
- <2 weeks after the mother received RSV vaccine during pregnancy
Nirsevimab is also recommended for the following infants after assessment by their treating doctor to confirm potential clinical benefit:
- infants with risk conditions for severe RSV disease, regardless of maternal vaccination (see Table. Conditions associated with increased risk of severe RSV disease in infants and young children)
- infants born to mothers with severe immunosuppression, where the immune response to maternally administered RSV vaccine was impaired (see People who are severely immunocompromised)
- infants who have lost effective passive immunisation:
- those whose mothers have received RSV vaccine in pregnancy but who have subsequently undergone a treatment after birth, such as exchange transfusion, cardiopulmonary bypass or extracorporeal membrane oxygenation, that may lead to loss of maternal antibodies, OR
- those who have already received nirsevimab but have subsequently undergone one of the procedures above (noting this would be a repeat dose of nirsevimab)
See Figure. Flowchart to guide which infants should receive nirsevimab in their 1st RSV season.
Nirsevimab is not recommended for infants during the first 6 months of life if:
- the infant’s mother received RSV vaccine at an appropriate time during pregnancy, AND
- the infant does not have a risk condition for severe RSV disease
| Risk category | Details or specific conditions |
|---|---|
| Preterm birth |
|
| Cardiac disease |
|
| Significant immunosuppression (individual conditions listed and those that are similar based on clinical judgement) |
Examples include:
|
| Chronic respiratory disease |
|
| Neurological conditions that impair respiratory function (individual conditions listed and those that are similar based on clinical judgement) |
Examples include:
|
| Chromosomal abnormality |
|
Figure. Flowchart to guide which infants should receive nirsevimab in their 1st RSV season
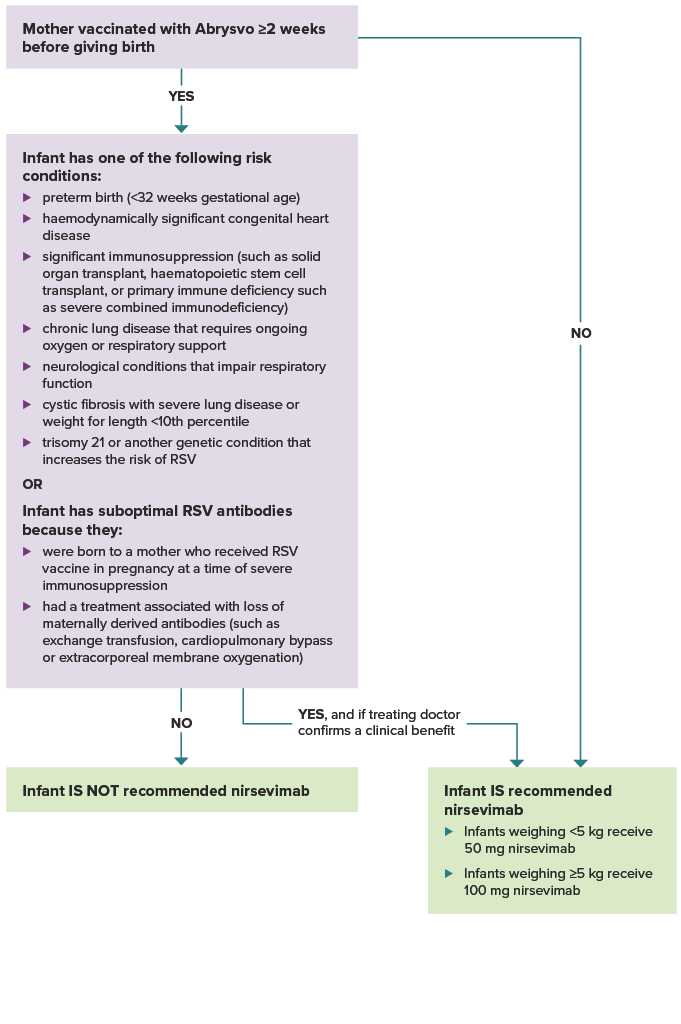
Was the mother vaccinated with Abrysvo at least 2 weeks before giving birth? If yes, and the infant does not have one of the risk conditions or conditions for suboptimal antibodies listed below, the infant is not recommended nirsevimab.
If the mother was not vaccinated at least 2 weeks before delivery, the infant is recommended nirsevimab. Infants weighing less than 5 kg receive 50 mg nirsevimab. Infants weighing 5 kg or more receive 100 mg nirsevimab.
If the mother was vaccinated at least 2 weeks before delivery, does the infant have one of the following risk conditions: preterm birth (<32 weeks gestational age), haemodynamically significant congenital heart disease. significant immunosuppression (such as from malignancy, solid organ transplant, haematopoietic stem cell transplant, or primary immune deficiency such as severe combined immunodeficiency), chronic lung disease that requires ongoing oxygen or respiratory support, neurological conditions that impair respiratory function, cystic fibrosis with severe lung disease or weight for length <10th percentile, trisomy 21 or another genetic condition that increases the risk of RSV? Or, does the infant have suboptimal RSV antibodies because they were born to a mother who received RSV vaccine in pregnancy at a time of severe immunosuppression, or they had a treatment associated with loss of maternally derived antibodies (such as exchange transfusion, cardiopulmonary bypass or extracorporeal membrane oxygenation)? If the infant does not have a risk condition or suboptimal RSV antibodies, they are not recommended nirsevimab. If the infant does have a risk condition or suboptimal RSV antibodies, they are recommended nirsevimab if their treating doctor confirms a clinical benefit.
Other general considerations on the use of RSV monoclonal antibody
Other general considerations on the use of RSV monoclonal antibody
- Infants <3 months of age in their 1st RSV season have a greater risk of severe disease than older children in all categories.
- Infants with multiple risk factors for severe RSV disease, such as preterm birth and a medical risk condition, are likely to have an even higher risk of severe outcomes.
- The risk of hospitalisation from RSV for Aboriginal and Torres Strait Islander infants is around 2 times that of non-Indigenous infants of the same age.7,8
- Infants who live in regions where advanced care for severe RSV is not readily accessible may have greater benefit.
- Palivizumab (short acting RSV mAB) may be available as an alternative RSV monoclonal antibody for eligible infants. Palivizumab is recommended for infants who have a risk condition (i.e. not for infants born to an unvaccinated mother). See Table. Conditions associated with increased risk of severe RSV disease in infants and young children. Palivizumab is given as up to 5 monthly injections from shortly before the start of the RSV season.
Timing of RSV-specific monoclonal antibodies in infants
Timing of RSV-specific monoclonal antibodies in infants
The timing of administration of monoclonal antibody should ensure that the duration and level of protection are maximised over the peak months of a child’s 1st RSV season. This is typically April to September in temperate regions of Australia, but this may vary for different regions. Local advice should be sought. Infants who have previously had an RSV infection who are recommended to receive RSV-specific monoclonal antibodies can receive it once recovered.
Nirsevimab offers protection for at least 5 months, with early immunogenicity evidence suggesting some protection may remain for 6-12 months.9 Protective benefits can be maximised if it is administered:
- shortly after birth for infants born just before or during the RSV season. For infants born after the RSV season, consider the likelihood of out-of-season RSV infection and risk of severe disease (see Table. Conditions associated with increased risk of severe RSV disease in infants and young children), and consider delaying nirsevimab until just before the next RSV season, if appropriate
- shortly before the start of their 1st RSV season in older infants that remain at high risk.
Infants and young children who are at risk of severe RSV disease and who have previously had an RSV infection and are recommended to receive RSV-specific monoclonal antibodies can receive it once recovered.
Nirsevimab is funded through state and territory programs for some infants and young children. See state and territory guidance for details of each program.

